
Cardiology Doppler Probe Supplier - Kody Medical
CARDIOLOGY
BLOOD FLOW VOLUME METER
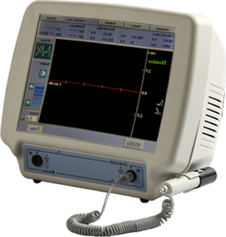
DVM 4500
Bi-directional Doppler with large colour touchscreen. Windows based simple touch operation. 2 probe combinations for 4, 5, 8, 10, 20MHz, & PPG. 30 waveform memory & USB interface. PDF and DICOM Export available for USB flash memory. Downsized design with high speed CPU & FFT analysis. PPG probe (Option)..
Features:
- 10-inch Large Colour Touchscreen
- High speed CPU & FFT Analyser
- Interchangeable probe 4, 5, 8, 10 and 20 MHz
- Light weight & Downsizing body
- Internal 30 waveform memory
Wave form mode
- Spectrum, Max & Mean
- ZCC (Zero Cross Counter)
Velocity range (FFT)
- 4MHz 5MHz 8MHz
- 6-240 5-200 3-120 [cm/s]
- 10MHz 20MHz
- 2.5-100 1.5-50 [cm/s]
Vessel diameter
- 0.1 to 20mm
- PPG options
- Exporting to PDF and DICOM files with USB flash memory
Application
Coronary Artery Bypass Grafting(CABG)
- Confirm blood flow after bypass surgery
Coronary artery bypass grafting(CABG)
- Abdominal Aortic Aneurysm(AAA)
Bypass surgery of the lower limbs
- (F-F bypass・A-F bypass・F-P bypass)
- F:Femoral artery A:Aorta P:Popliteal artery
SURGICAL PROBES

NRP PROBE 10MHz/20MHz
NRP 10MHz/20MHz Surgical probe is compatible with Hadeco Bidirectional Dopplers Minidop ES100VX, Bidop ES100V3, Smartdop45.

CRP PROBE 10MHz/20MHz
CRP 10MHz/20MHz Surgical probe is compatible with Hadeco Bidirectional Dopplers Minidop ES100VX, Bidop ES100V3, Smartdop45.
VRP PROBE 10MHz
VRP 10MHz Surgical probe is compatible with Hadeco Bidirectional Dopplers Minidop ES100VX, Bidop ES100V3, Smartdop45.

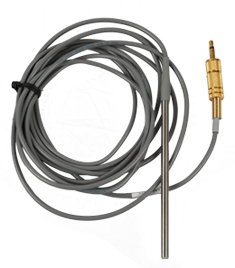
TUCKING PROBE 10MHz (3,4,5mmDia)
Surgical Tucking probe 10MHz (3,4,5mmDia) is compatible with Hadeco Bidirectional Doppler Blood Volume flow meter Model DVM 4500.

NP PROBE 10MHz flexible (2mm Dia)
NP10M2S8AF-2mm Dia flexible surgical probe is compatible with Hadeco Bidirectional Doppler Blood Volume flow meter Model DVM 4500.

NP PROBE 20MHz flexible (1.2mm Dia)
NP20M12S8AF-1.2mm Dia flexible surgical probe is compatible with Hadeco Bidirectional Doppler Blood Volume flow meter Model DVM 4500.

NP PROBE 20MHz Bayonet Ultra-thin (0.8mm Dia)
NP20M1S8A-0.8mm Dia flexible surgical probe is compatible with Hadeco Bidirectional Doppler Blood Volume flow meter Model DVM 4500.
STERILISATION PROCEDURE
METHOD 1 (ETO)
CLEANING
Probes must be cleaned to remove contaminating substances prior to sterilization. After each use, perform initial cleaning within the sterile field by wiping the probe free of blood and body fluids. Then, pass the probe out of the field. Disconnect the probe (and any adaptor) from the Doppler unit, clean the probe tip and housing thoroughly with a mild soapy solution, a soft cloth with an enzymatic cleaning solution, or an alcohol-based wipe. If using an enzymatic cleaning solution, wipe the probe again using mild soapy water or an alcohol-based wipe. Do not soak. Complete preparation for sterilization in accordance to hospital procedure using an approved probe cleaning agent.
STERILIZATION 80%
It is recommended that the intraoperative and laparoscopic probes be gas sterilized before each use with ethylene oxide (ETO) gas. The suggested mixture ratio is ETO gas – 20%, CO2 gas – 80%. Exposure time should not be less than 1 hour and 45 minutes at a pressure of 0.75 to 0.8Kg/cm2 and a temperature of 50° to 60° C. To achieve a Sterility Assurance level (SAL) of 10-6 (the recommended minimum for direct contact with blood), an exposure time of 3 hours and 30 minutes is necessary. A biological indicator strip should be placed in the load to verify the effectiveness of the cycle selected.
WARNING
Do not sterilize the Doppler probes listed above by steam autoclaving. Steam sterilization is likely to damage the plastic probe tip and piezo-electric crystals. Utilize Koven/Hadeco probe number ACP-08 for steam autoclaving. Do not use an automatic washer/disinfector. Temperatures will cause probe electronics to melt.
AERATION
Follow hospital requirements with regard to aeration time to allow residual ETO gas to dissipate. The minimum recommended aeration time is 8 hours at 60° C, 40% to 60% humidity with an air change (ventilation) frequency of 25 times per hour. Note: aeration time should meet institution and FDA guidelines.
AERATION
During the examination, fluid coupling must be maintained for proper signal reception. If needed, irrigate the incision or laparoscopic portal with sterile saline to insure adequate coupling. Use of sterile gel is acceptable in all areas except the brain.
CONTRAINDICATIONS: Hospital policy takes precedence over this protocol.
Failure to use approved cleaning & sterilization procedures may void the warranty
METHOD 2 (STERRAD)
CLEANING
Probes must be cleaned to remove contaminating substances prior to sterilization. After each use, perform initial cleaning within the sterile field by wiping the probe free of blood and body fluids. Then, pass the probe out of the field. Disconnect the probe (and any adaptor) from the Doppler unit, clean the probe tip and housing thoroughly with a mild soapy solution, a soft cloth with an enzymatic cleaning solution, or an alcohol-based wipe. If using an enzymatic cleaning solution, wipe the probe again using mild soapy water or an alcohol-based wipe. Do not soak. Complete preparation for sterilization in accordance to hospital procedure using an approved probe cleaning agent.
STERRAD STERILIZATION SYSTEM GUIDELINES
The probes listed above may be sterilized with the Sterrad 100S, Sterrad 200, Sterrad NX and/or Sterrad 100NX (where indicated) sterilization systems (see charts above). The general processing instructions in the “Sterrad 100S, Sterrad 200, Sterrad NX and/or Sterrad 100NX Sterilization System Operator’s Manual(s)” should be followed. Probes must be cleaned to remove contaminating substances prior to sterilization. After each use, perform initial cleaning within the sterile field by wiping the probe free of blood and body fluids.
STERILIZATION
Per recommended Sterrad 100S, Sterrad 200, Sterrad NX and/or Sterrad 100NX procedures using a Sterrad brand or Sterrad compatible trays.
WARNING
Sterilize according to instructions for the Sterrad 100S, Sterrad 200, Sterrad NX, and/or Sterrad 100NX Model(s). Do not sterilize the Doppler probes listed above by steam autoclaving. Steam sterilization is likely to damage the plastic probe tip and piezo-electric crystals. Utilize Koven/Hadeco probe number ACP-8 for steam autoclaving. Do not soak or submerge the probe in liquid. Do not use Cidex. Do not use an automatic washer/disinfector. Temperatures will cause probe electronics to melt.
Failure to use approved cleaning & sterilization procedures may void the warranty.

ABPM50
Introduction
ABPM50 is a handhold ambulatory blood pressure monitor, which is designed according to oscillography theory. The device could monitor human body blood pressure up to 24 hours continuously and dynamically, providing accurate basis for the diagnosis. It is applicable for using in hospital, clinic and other medical institutions.
Main Features:
- Compact and portable, user-friend interface, easy to use
- Patient range: adult, pediatric, neonate
- 24 hours ambulatory NIBP monitoring function, up to 350 groups of ambulatory NIBP data can be recorded for once.
- Perfect combination of automatic and manual measurement method, up to 300 groups of data can be recorded for once by manual measure.
- High-definition color TFT display, strong visibility
- By data review interface such as “data list”,”trend graph”,”big font”, NIBP data is clear at a glance
- Display of low power prompt, alarm, error message and time
- Supply two kinds of unit: mmHg / kPa
- Parameter alarm dispose function is optional
- Communicate with PC, PC software can achieve data review, measured results analysis, view of trend graph, reports printing and other functions
Software features:
- Connect to the device by USB interface.
- Download NIBP measure result from the terminal device.
- Display of scoop-shape trend graph, filling-type trend graph, histogram, pie chart, correlation line graph.
- Edit every piece of NIBP data, and add annotation to it.
- Edit basic information, doctor’s advice, NIBP status instruction, current medicine-taken information, etc.
- Support report printing and print preview.
 kodymedical@gmail.com
kodymedical@gmail.com +91-9380223396
+91-9380223396 My Account
My Account
